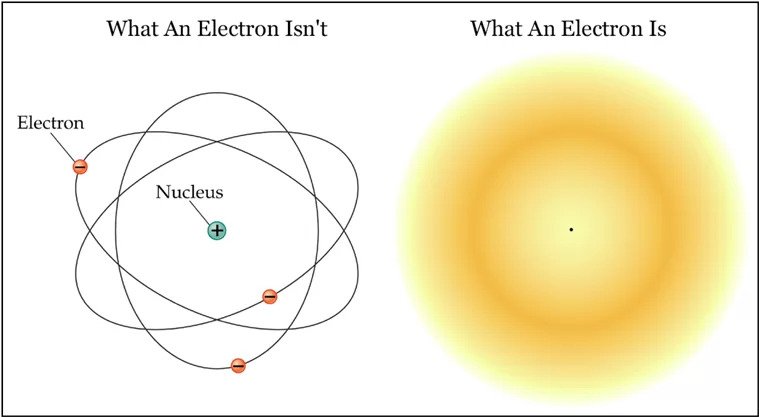
The Erwin–Schrödinger Electron Cloud model, also known as the Schrödinger wave equation, was developed in 1926 to describe the behavior of electrons in atoms. Schrödinger proposed to describe electrons as waves existing in three-dimensional space around the nucleus.
This model assumes that electrons move along paths described by potential distributions around the nucleus or by electron clouds rather than specific paths.
Limitations:
These models revolutionized our understanding of atomic structure by enabling scientists to more accurately predict the behavior and properties of electrons in atoms.
A mathematical model explains how electrons expand in orbitals or energy levels around the nucleus using mathematical formulas. Schrödinger’s work paved the way for the development of modern quantum mechanics.
Some Explained Drawbacks of Electron Cloud
Quantum mechanics today describe the behavior of matter at the atomic and subatomic levels.
Electron cloud models have come a long way, but they have some limitations. The main disadvantage of this model is that it assumes that the electron is at rest. However, electrons in atoms are in constant motion, and it is difficult to accurately predict these movements.
Furthermore, this model does not consider the effects of external forces on the electron’s behavior, such as the effect of an applied electric or magnetic field.
In such cases, the model becomes difficult to implement and requires more sophisticated techniques to predict the electron’s behavior.
Electron Cloud Model Vs. Quantum Mechanical Model:
A quantum mechanical model and an electron cloud describe electron behavior in atoms, but they differ in some important ways.
The quantum mechanical model is a refined version of the electron cloud that takes into account the wave-particle duality of the electron.
It describes the behavior of electrons in a mathematical formula called a wave function.
It predicts the probability of finding an electron at a given location. A quantum mechanical model, unlike an electron cloud, can apply to molecules and solids.
How does the electron cloud model describe electrons?
It describes electrons as existing in energy levels or orbiting the nucleus. Quantum numbers describe the shape, size, and direction of these orbitals.
This model assumes that electrons are in a stochastic cloud that represents the probability of finding an electron at a particular location. When electrons and positively charged nuclei interact, they form an electron cloud. Electrons exist in four main types of orbitals.
Despite some limitations, the model was of great importance in the development of modern atomic theory and in understanding how matter behaves at the atomic and subatomic levels.
Conclusion
You Read This Article On Examviews.com where You can Get All the Latest Updates, News, and Reviews.
What do you think about the post? Leave a comment below.
